 Global Operation
Global Operation

JMS contributes to better health and QOL for all people around
the world with the supply of medical devices promptly and stably.
“Provide required devices to medical field” is our spirit and it is unchanged even after our global operation is developed.
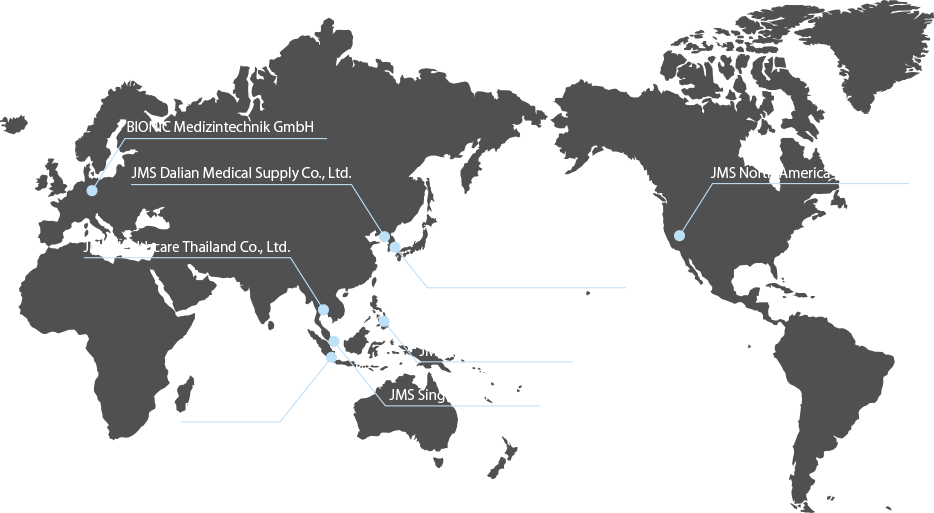
We started to market products to overseas in the 1970s and are currently supplying to more than 90 countries.
With close collaboration with the affiliated companies in the world,
we will further strengthen our brand power, aiming to penetrate the global market with the “JMS” brand.
Target of our global business
In order to grow the business of expanding medical demands mainly in emerging countries,
we have established Global Marketing Division. For accelerating its global business,
we aim to advance “development and production to satisfy actual needs,”
which is the business model of Japan domestic business,
and expand the business by facilitating communication among the group companies in the world.
Promotion of optimized
production and marketing
Development and Production to satisfy local actual needs
It is necessary to gain the abilities like; gathering information of each regional inputs precisely, technical capability to reflect such inputs into the development and the production and marketing network to distribute the outputs as products in order to deliver the required products to the medical field. Therefore, we exchange information and shares technology through active personnel interchange among the group companies and advances deployment of products that fit the needs and trends in each country or region. To fulfill the mission of stably supplying high-quality products, we have prepared a system for “self-development/production” and is proactively making efforts to obtain international certification.
-
Create safe and
user-friendly productsTechnical development

With our “internal development organization”, we consistently integrate in-house processes from prototype making and created many own products and technologies. With this experience, we can deliver required products promptly to medical fields corresponding to each request.
-
Maintain stable quality
and supply of medical devicesQuality Control

We establish “internal production organization” where the processes from material preparation to the final sterilization of products. We also have a management system compliant with the Japanese PMD Act (Pharmaceutical and Medical Device Act) and American/European regulatory requirements and has obtained ISO13485 certification, international standards of quality management exclusively for medical devices.
Expansion of business domains
-

Enhancement of competitiveness and sales capabilities in ASEAN countries
Recently, there has been an increasing demand for medical devices mainly in emerging countries, such as Southeast Asian countries. In response to this increase, we will broaden our product line and expand our sales offices for further growth, making use of the advantage for having production sites in Asian countries like Indonesia and the Philippines. In China, a rapidly growing market, we are developing its core business, in addition to its currently growing central dialysis fluid delivery system (CDDS) business.
-
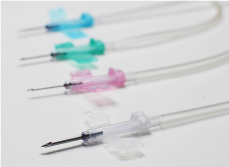
Promotion of OEM*business
We are stably delivering high-quality products to the global market through its contract manufacturing companies, mainly for arteriovenous fistula (AVF) needles and transfusion products, and hold a dominant market share. We have the advantage of providing customers with total support, satisfying legal requirements in global markets and supporting BCP*risk control with various JMS group operations as well as the ability to design and develop products. We plan to expand OEM business more by utilizing our own strengths.
*The Original Equipment Manufacturer (OEM) handles the manufacturing of products belonging to the brand of another company on a contract basis.
*Business Continuity Plan (BCP)

