

Providing Solutions
to Support
Regenerative
Medicine
Incorporating innovations including the discovery of induced pluripotent stem cells (iPS cells), regenerative medicine is expected to advance dramatically in clinical application. While demand for pioneering therapies and drug discoveries is rising, there are also growing needs for higher technological advancement to assure safety of delicate processes such as culture and transplantation of cells. We, at JMS, will provide devices that support regenerative medicine and anticipate future clinical applications, by utilizing our experience and technologies in the development and manufacture of devices requiring high levels of safety, including blood bags and other supplies.

Enable technological innovation in medical care with
our unique know-how on maintaining a suitable
environment supporting survival of blood and cells.
-
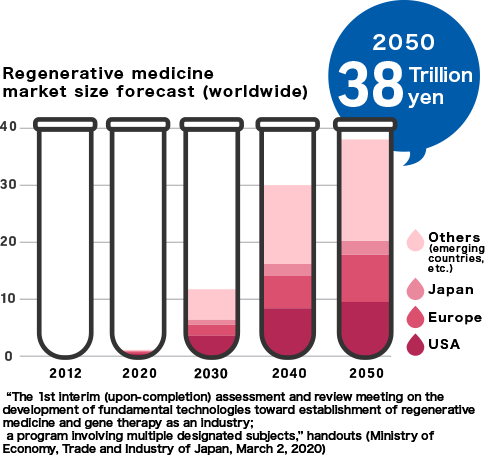

While medical technologies have developed impressively worldwide, many illnesses and injuries still remain difficult to treat. These problems are expected to be overcome by utilizing technologies based on regenerative medicine. The Japanese market, valued at 95 billion yen in 2020, is projected to grow to 2.5 trillion yen in 2050, and the global market is projected to reach 38 trillion yen.
-
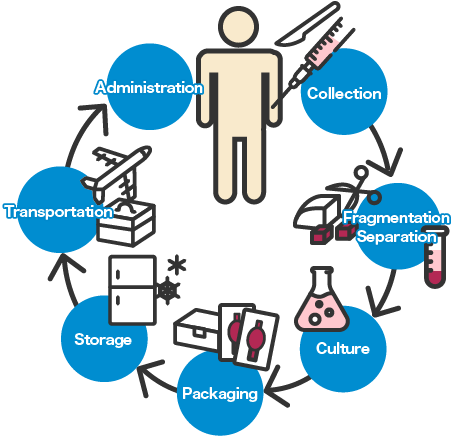

To establish regenerative medicine, development of various technologies relating to cell processing, such as cell culture, grafting, transportation, and storage, is crucial. Currently, technologies of not only the medical industry but also of various other industries are being introduced to ensure the maintenance of necessary environment and devices, allowing the strengths of each field to be applied to clinical application.
-
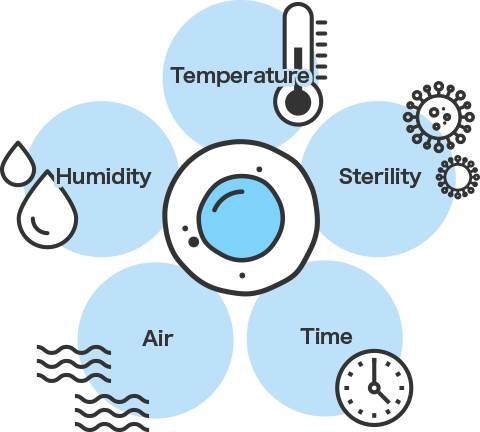

The key is the container in which cells dwell for a long time. “Viable” cells play an active role in regenerative medicine. High levels of safety are required from containers used in all processes, from cell collection to grafting. Of course, maintaining sterility is also important, and establishment of regenerative medicine requires extremely high levels of quality in terms of both cleanliness and safety.
JMS Idea
Always obtain the latest data through industry-government-academia collaboration, to develop products related to all processes of regenerative medicine.
JMS has been engaged in joint development with doctors as well as collaborations with organizations such as universities, research institutions, and start-up companies, maximizing its strengths in medical device development in the field of regenerative medicine where high levels of safety are required. We appropriately identify needs in each setting to gain additional insights for product development. We leverage our unique technologies to promote clinical application of regenerative medicine.
Product
-
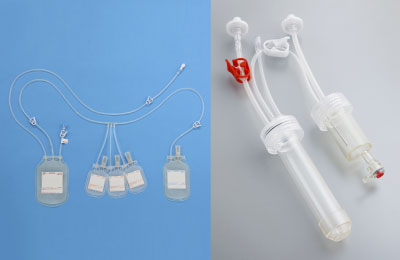
Blood component separation bag
A device for preparing sera and plasma containing platelets and other components from collected blood. The device provides high levels of airtightness and closed environment for preparation, separation, and storage, thereby reducing risks of microbial contamination. The device is also used in the “procedure to prepare platelet rich plasma (PRP)” on NHI price listing.
-
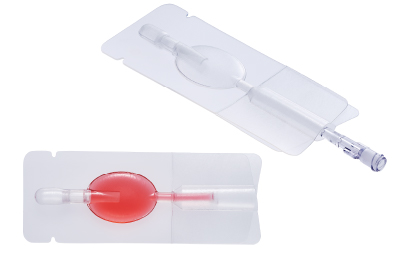
Cryopreservation container
The material used in this device is flexible and withstands freezing, enabling storage of cells at -196°C. With heat-welded tubing, the container assures a high level of safety in the process of sealing the container and storing the cells.



